M. Forjan defended his PhD thesis
02/03/2023Mateo Forjan defended his PhD thesis “Ultrafast Spectroscopy and Photochemistry of Intermediates With Potential Application in Biology and Medicine”.
Thesis presents research of the photochemistry and ultrafast dynamics of quinone methides – intermediates with potential applications in biology and medicine. For the purpose of conducting research, an experimental setup for ultrafast transient absorption was built at the Institute of Physics in Zagreb. A time resolution of 200 fs was achieved and the pump pulse wavelengths of 267nm, 400nm or 800nm can be chosen. The following quinone methide precursors were selected: adamantyl phenol, adamantyl and non-adamantyl derivatives of naphthol, and BODIPY-phenol. It was discovered that from adamantyl-phenol, quinone methides are formed in the ground state in an ultrafast photodehydration reaction through conical section with a time constant of 100fs. With naphthol derivatives, it has been observed that although the molecules share a chromophore, the reactions differ greatly. In the case of adamantane naphthol, the quinone methide is also formed in the basic state through a conical cross-section, but more slowly, on a nanosecond scale, while in the case of naphthol derivatives without the adamantyl part, it is formed after 40μs by the elimination of the OH· group from the radical of the precursor molecule, which is formed either from the radical cation or from the S1 state. Time-resolved fluorescence measurements of BODIPY-phenol confirmed the anti-Kasha physics of the sample.
The scientific contribution of the work lies in the fully elucidated photochemical reactions of quinone methide formation from three precursors. Quinone methides are important intermediates in the chemistry and photochemistry of phenols and are interesting because of their biological activity and increasing application in organic synthesis. Their biological activity is based on antiproliferative action, which is why they are applicable in photochemotherapy.
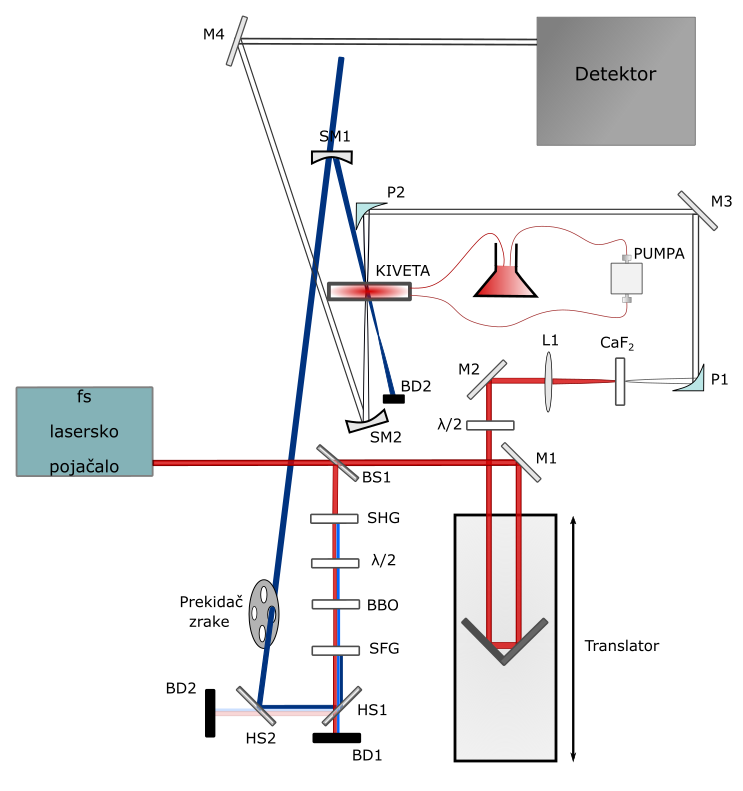
Ultrafast transient absorption experimental setup at Institute of Physics in Zagreb. M = mirror, BS = beam splitter, L = lens, P = parabolic mirror, SM = spherical mirror, HS = harmonic separator, BD = beam dump, SHG = second harmonic generation, SFG = sum frequency generation.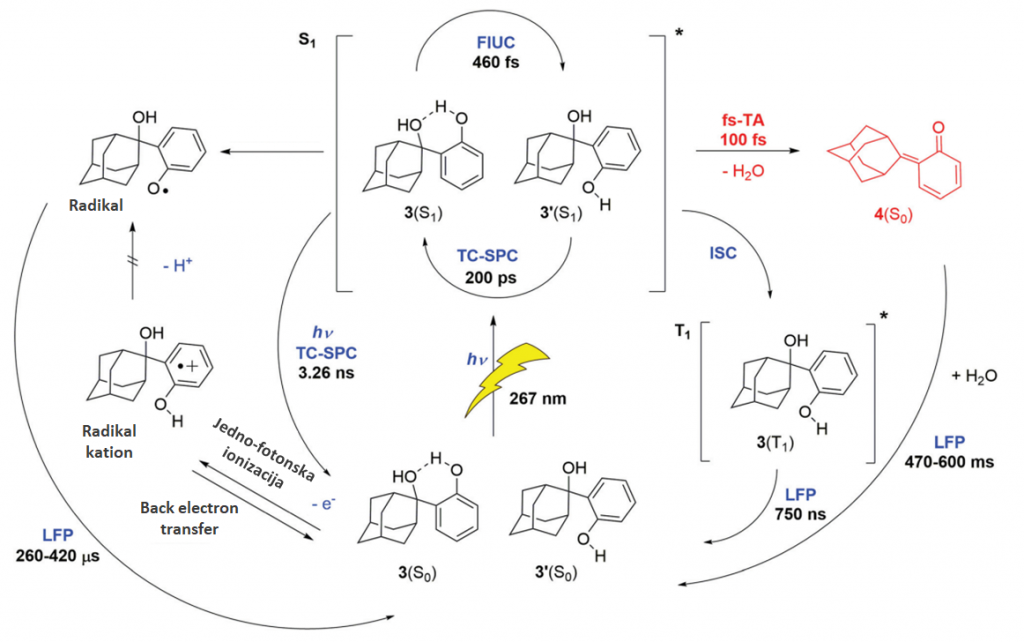
Complete photochemical reaction pathway for adamantyl phenol after excitation to S1 state. Quinone methides are generated in its ground state in 100fs by photodehydration. Besides quinone methides, triplet states, radicals and radical cations are produced.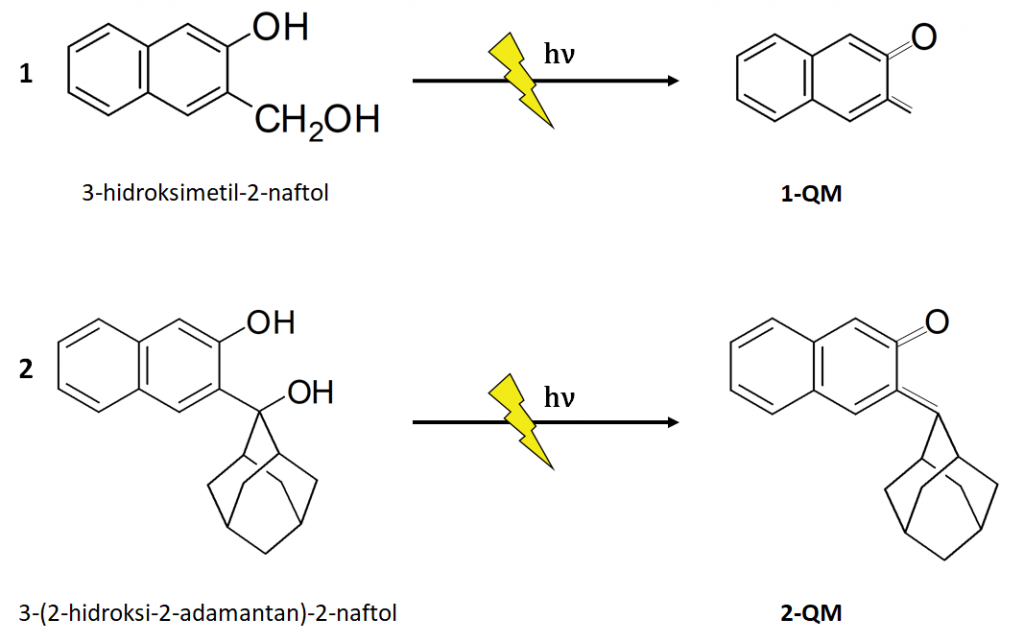
2-naphthol derivatives also used as a precursors for QM generation. Besides sharing the same chromophore (2-naphthol), pathways of photochemical reactions differ greatly.
 Group for Applied Ultrafast Spectroscopy
Group for Applied Ultrafast Spectroscopy